When it comes to matters of science the make-up of substances matters. Transition metals (minerals) affect all the processes in living things. Zinc for example, has profound impacts in processes affecting transcription1, cellular proliferation2, oxidative stress3, immunity4,5, and blood pH balance6, and specifically in plants zinc plays a key role in bud formation, leaf formation and leaf size. Making this mineral important to plants, animals and people. Zinc must reach specific areas inside living things but may have a hard time moving without help, especially in plants as it is considered one of several non-mobile minerals.
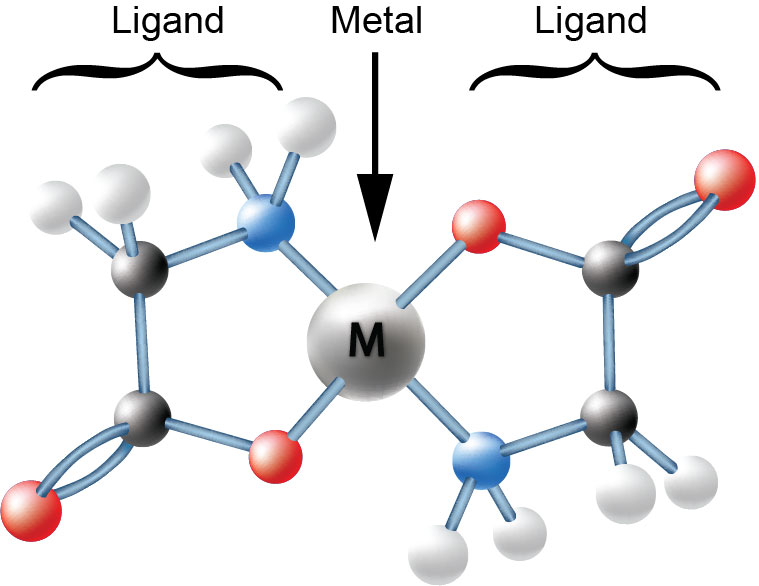
That is where chelation comes in. Morgan and Drew7 initially coined the term chelate when they observed that organic molecules form bonds with metallic atoms and hold it at two points, like a claw. Chelates are common in nature, including hemoglobin and chlorophyll, with chlorophyll being a chelated form of magnesium8. A chelate is characterized by its unique structure as the organic material bonds with the mineral in more than one place. This allows the organic molecule to protect the mineral.
When using a chelated mineral for supplementation or fortification, bioavailability is significantly impacted by how the bond is formed with the metal. Giroux and Prakash’s study on rats showed this as they observed zinc was able to be more effectively translocated with chelates versus minerals alone.9 Also, of crucial importance is the stability of the bonds, so plants can effectively access the minerals.
It is critical that when minerals are applied to plants the minerals must be in a form that is bioavailable. Chelated molecules exhibit different attributes, and higher bioavailability than either the metal or ligand alone. Balchem’s Metalosate® products, powered by Albion® Technology, contain Amino Acid chelates which possess a specific chemical structure that has demonstrated increased bioavailability in human, animal, and plant applications. For an in-depth analysis of the scientific principles behind chelates, bioavailability, and the structure of chelated minerals take a look here.
References:
- Deficiency of the zinc finger protein ZPR1 causes defects in transcription and cell cycle progression. Gangwani, L. 2006, The Journal of Biological Chemistry, Vol. 281, pp. 40330-40340.
- The zinc sensing receptor, a link between zinc and cell signaling. Hershfinkel, M., Silverman, W. and Sekler, I. 2007, Molecular Medicine, Vol. 13, pp. 331- 336.
- The function of zinc metallothionein: a link between cellular zinc and redox state. Maret, W. 2000, The Journal of Nutrition, Vol. 130, pp. 1455S1458S.
- Zinc and the immune system. Rink, L. and Gabriel, P. 2000, Proceedings of the Nutrition Society, Vol. 59, pp. 541-552.
- Zinc in human health: effect of zinc on immune cells. Prasad, A. 2008, Molecular Medicine, Vol. 14, pp. 353-357.
- Apo-human carbonic anhydrase II revisited: implications of the loss of a metal in protein structure, stability and solvent network. Avvaru, B., et al. 2009, Biochemistry, Vol. epub ahead of print.
- CLXII.—Researches on residual affinity and co-ordination. Part II. Acetylacetones of selenium and tellurium. Morgan, G. and Drew, H. 1920, The Journal of the Chemical Society Transactions, pp. 1456-1465.
- Voet, D. and Voet, J. Biochemistry, third edition. Hoboken: John Wiley & Sons, Inc. 2004. P 321,
- Influence of zinc-ligand mixtures on serum zinc levels in rats. Giroux, E. and Prakash, N. 1976, Journal of Pharmaceutical Sciences, Vol. 66, p. 391